Adjunct Treatments for Dry Eye Disease
An overview of selected products and their uses for the management of ocular surface disorders.
By Callan Navitsky, Senior Editor, CRST Europe; With Expert Commentary From Ahmad M. Fahmy, OD, FAAO, Dipl ABO; P. Dee G. Stephenson, MD; Jodi I. Luchs, MD; Cynthia Matossian, MD; Rohit Shetty, FRCS, PhD; Harsha Nagaraja, MS, FCE; and James S. Lewis, MD
Avenova Daily Lid and Lash Hygiene

Figure 1. Avenova lid and lash cleanser.
Avenova Daily Lid and Lash Hygiene (NovaBay; Figure 1) is specifically designed for patients with blepharitis, meibomian gland dysfunction (MGD), and chronic dry eye disease (DED). Avenova functions by clearing debris and microorganisms from eyelids and eyelashes and by reducing inflammation. It is ideal as part of any lid hygiene regimen for DED, blepharitis (including Demodex), after make-up removal, contact lens wear, and ocular surgery and procedures, according to company literature.1 Avenova contains Neutrox, a pure, proprietary, stable formulation of hypochlorous acid (0.01%), the same molecule used by white blood cells to inactivate pathogens.AT A GLANCE
• Avenova lid and lash cleanser clears debris and microorganisms from eyelids and eyelashes and reduces inflammation.
• In the BlephEx procedure, a medical-grade microsponge is spun along the edge of the patient’s eyelids and lashes, removing scurf and debris.
• Cliradex—an all-natural lid, lash, and facial cleanser—contains isolated 4-Terpineol, the most important ingredient found in tea tree oil.
• The LipiFlow Thermal Pulsation System, an inner and outer eyelid thermal pulsation treatment, directly treats and unblocks the meibomian glands.
• MiBo Thermoflo employs a proprietary thermoelectric heat pump to help maximize liquefaction of meibum, improving preservation and function of the tear film’s evaporative component.
In vitro studies of Avenova demonstrated an antimicrobial kill time of less than 1 minute. Studies have confirmed Avenova’s broad-spectrum activity against microorganisms, including those most commonly found on eyelids and eyelashes: Serratia marcescens, methicillin-resistant Staphylococcus aureus, Staphylococcus epidermis, and Staphylococcus haemolyticus. Avenova has also been shown to be effective against Demodex.
Because it is well tolerated by patients and easy to use, Avenova can be used as a preventive measure and can be incorporated into a patient’s daily lid and lash hygiene regimen. Patients with blepharitis and other serious lid and lash conditions have seen results with Avenova in 2 weeks, according to the company. Marked decreases in dryness, redness, itching, and burning have been reported. Soothing and refreshing, Avenova improves patients’ compliance. It does not have any contraindications or side effects, so patients can feel comfortable using it every day.
Avenova is available in 40-mL bottles and requires a prescription. The recommended regimen is twice daily for
2 weeks or as directed by the physician.
1. Avenova. NovaBay. http://avenova.com/. Accessed March 18, 2016.
IMPROVING OSD AND FACILITATING PHYSICIAN COLLABORATION WITH AVENOVA


By Ahmad M. Fahmy, OD, FAAO, Dipl ABO
I practice in a high-surgical-volume, tertiary-care private practice as the director of optometric services. Minnesota Eye Consultants is an integrated practice, using a model of collaboration between ophthalmology and optometry pioneered by Richard L. Lindstrom, MD, and others. After completing a comprehensive fellowship program, I became responsible for perisurgical care of cataract, refractive, glaucoma, and oculoplastics cases in addition to my nonsurgical patients. Over the years, I have developed a special interest in ocular surface disease (OSD) and anterior segment pathology. I am especially pleased to have the opportunity to comment on what has quickly become a key component of my OSD baseline treatment protocol: Avenova eyelid cleanser.
The majority of my patients have one form or another of blepharitis. Although it is rather easily identified, pinning down all contributing factors and developing a treatment plan that sufficiently addresses these factors are complex, as the interactions of overlapping conditions can often intensify blepharitis. Skin conditions closely related to chronic blepharitis include facial rosacea, eczema, seborrhea, and acne. Additional systemic conditions and factors closely related to ocular surface inflammation may include glaucoma drop toxicity, autoimmune disease, contact lens use, allergy, and environmental challenge.
When more than one of these inflammatory culprits (by no means a comprehensive list) affects the same eye, a carefully thought out and robust protocol for identifying the inflammatory components and effectively treating them becomes a necessity. In my experience, if the trigger is properly and specifically treated, the inflammatory imbalance can be corrected. The earlier the patient is in the inflammatory cycle, the better the chance of successful treatment is.
For these reasons, I have been enthusiastic about the addition of an eyelid cleaner that is proven to be antimicrobial, anti-inflammatory, and nontoxic to the ocular surface. I have been recommending eyelid hygiene with pure hypochlorous acid for several years with terrific results. Before Avenova, I did not have a go-to eyelid hygiene product that I could rely on as a scientifically proven antimicrobial and anti-inflammatory. Our recommendations for blepharitis—from baby shampoo (a detergent) to bacitracin (a medication that many patients might be sensitive to)—have varied due to a lack of evidence.
The group of patients for whom Avenova seems to have the most impact is the seborrheic blepharitis group. I find that, when I instruct patients to apply it using a cotton-tipped swab, a larger amount of debris is removed, the lids are less inflamed, and, most important, the patient’s subjective feedback on the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire is significantly improved. Of course, this is not something I can recommend to every patient; there are many factors that might preclude a patient from using a cotton-tipped swab safely. I also heavily prescribe Avenova for patients undergoing any ocular surgery.
Avenova is a terrific addition to the basic OSD treatment plan and can be a tool that facilitates collaboration among ophthalmologists and optometrists in the best interest of our patients.
Ahmad M. Fahmy, OD, FAAO, Dipl ABO
• director of optometric services, Minnesota Eye Consultants, Bloomington, Minnesota
• amfahmy@mneye.com
• financial disclosure: member of advisory board for NovaBay
Avenova: A VALUED ADDITION TO MY PRACTICE









By P. Dee G. Stephenson, MD
DED and blepharitis are two of the most common reasons patients seek my help. Blepharitis is one of the most common eye diseases that we treat and is one of the big culprits when dealing with OSD and DED and preoperative surgery patients. Signs and symptoms of blepharitis run the gamut, from itchy and sore red eyelids; to stuck-together eyelids, burning, and gritty feelings; to photophobia, swollen lids, and eyelash loss. With complications of not only DED but also conjunctivitis and MGD, corneal damage may occur. Many of these patients have seen other doctors without relief of these conditions that affect daily life. Some patients have had these conditions for many years without relief, and some have given up reading and other hobbies.
In patients with DED and blepharitis, the first step is to examine their lids and look for underlying causes. We all know that patients’ own flora of bacteria is often the culprit. However, there are other potential causes as well such as Demodex mites, which burrow into the follicles along the lash line. The mites’ waste builds up on the eyelids and causes inflammation and redness, and this debris can clog the meibomian glands, which decreases production of oil—an important part of the tear film. Patients then experience all of the dry eye symptoms. The mites can also affect some of the bacteria that colonize the eyelids, releasing toxins and causing more symptoms to occur such as inflammation, pain, and discomfort.
To date, the mainstay treatments for this issue have been hot compresses, lid scrubs, and ophthalmic ointment. For mites, although I have sometimes used tea tree oil, there is no standard of care for this, and it can also irritate the eye. It is important to recognize and treat these conditions the best we can. The good news for my practice and patients came about 1.5 years ago when I implemented Avenova with Neutrox.
Avenova does not generate resistance; it removes bacteria and Demodex. It has antibiofilm and antitoxin activity. It is active against a broad range of pathogens and is effective against methicillin-resistant S aureus, S aureus, S epidermidis, S haemolyticus, and serratia marcescens. Avenova is very fast acting: it kills 99.9% of these offenders in 60 seconds.
Avenova has been valuable to my practice and has eliminated my use of bacitracin, polymyxcin, tobradex, neomycin, loteprednol, and even oral doxycylin. I also use Avenova pre- and intraoperatively to protect surgical patients from endophthalmitis. It is easy to use: simply apply to a cotton
pad, wipe the lids and lashes from side to side, and let air dry. This should be done two times a day, and in about 2 weeks, the symptoms and health of the eyelids will improve. Avenova can be used indefinitely and continues to allow patients a much better option than other current treatments. Most important, their eyes feel better, so they feel better. Happy patient, happy doctor!
P. Dee G. Stephenson, MD
• president, American College of Eye Surgeons (ACES)
• founder, Stephenson Eye Associates, Venice Florida
• eyedrdee@aol.com
• financial interest: none acknowledged
BlephEx In-Office Treatment
BlephEx (RySurg; Figure 2) is an in-office procedure for the treatment of DED and blepharitis. BlephEx removes excess bacteria, biofilm, and bacterial toxins, the main causes of inflammatory dry eye and lid disease.
According to company literature,1 the BlephEx treatment is a painless procedure, during which an eye care professional uses the BlephEx handpiece to precisely and carefully spin a medical-grade microsponge along the edge of the patient’s eyelids and lashes, removing scurf and debris. The procedure lasts about 6 to 8 minutes, and the eyes are rinsed well afterward.


Figure 2. The BlephEx device.
The BlephEx treatment is well tolerated by patients, most of whom simply report a tickling sensation, according to the company. A numbing drop is typically placed in each eye prior to treatment for increased patient comfort.
After the treatment, the patient is instructed on how to maintain his or her clean eyelids with regular nightly lid hygiene. Because home treatments are only partially effective, the procedure is typically repeated at 4- to 6-month intervals, according to RySurg. The company recommends using the LidHygenix foam lid cleanser (LidHygenix) in conjunction with the BlephEx system.
1. BlephEx … The First and Only Doctor Treatment for Dry Eye and Blepharitis. RySurg. http://www.rysurg.com/index.php. Accessed March 13, 2016.
BlephEx: An Important Addition to the DED/OSD Practice









By Jodi I. Luchs, MD
Microblepharoexfoliation, or the BlephEx procedure, has been an important addition to my DED and OSD practice. The BlephEx procedure quickly and efficiently accomplishes several important objectives for patients. First, the procedure efficiently scrubs the lid margin, removing crusting, scurf, and collarettes from the base of the lashes. Coupled with use of an antibacterial foam, the bacterial load on the lid margin is reduced.
Additionally, the mechanical debridement efficiently removes the biofilm laid down by the lid margin bacteria, effectively eliminating the scaffold on which the bacteria live and grow. The result is a significant reduction in lid margin inflammation after the procedure—inflammation that frequently extends onto the ocular surface, where it contributes to OSD such as DED.
Another benefit of the BlephEx procedure is its effect on the meibomian glands. By extending the BlephEx procedure to the edge of the lid margin where the orifices of the meibomian glands reside, one can effectively unroof obstructed, capped, or inspissated glands, allowing meibomian gland secretions to again flow freely into the tear film. Additionally, the mechanical massage of the lid margin that occurs during the procedure may help to express the glands. As a result, this procedure provides an important benefit for patients with DED by improving tear film stability and helping to reduce the evaporative component of DED.
Jodi I. Luchs, MD
• codirector, Department of Refractive Surgery, North Shore/Long Island Jewish Health System, Great Neck, New York
• assistant clinical professor, Hofstra University School of Medicine, Hempstead, New York
• director of clinical research and director of cornea and external diseases, South Shore Eye Care, Wantagh, New York
• jluchs@aol.com
• financial interest: none acknowledged
Cliradex All-Natural Cleanser


Figure 3. Cliradex lid, lash, and facial cleanser.
Cliradex (BioTissue; Figure 3) is an all-natural, preservative-free lid, lash, and facial cleanser for the management of symptoms associated with blepharitis, MGD, rosacea, DED, Demodex infestation, chalazia, and other lid margin diseases.
Unlike traditional cleansers derived from manmade chemicals, Cliradex provides a natural way for patients to keep their eyelids and skin clean, comfortable, and healthy, according to company literature.1 The proprietary, all-natural formulation in Cliradex is safe for everyday use as part of a regular ocular and facial hygiene regimen.
Cliradex is derived from key constituents of Melaleuca alternifolia, a variety of tea tree oil. More than 20 years of research has reportedly been conducted to develop and support the effectiveness of Cliradex for OSD. Cliradex is the only commercially available product that isolates 4-Terpineol, the most important ingredient found in tea tree oil. 4-Terpineol has been demonstrated to safely and effectively kill Demodex mites better than tea tree oil and other ingredients.
Cliradex with 4-Terpineol is delivered on a soft, single-use towelette, which patients can purchase online or through their eye care providers. BioTissue also offers the Cliradex Complete Advanced Hygiene Kit, which consists of a formulation for in-office application that boasts a stronger concentration of the isolated 4-Terpineol than in the Cliradex for home use.
Cliradex can provide immediate relief through a cool, tingling, menthol-like feeling. Within days, mites begin to disappear, and patients’ eyes feel relief, according to the company.
1. Cliradex. http://www.cliradex.com/physicians/. Accessed March 11, 2016.
The Implications of Eyelid Disease on the Ocular Surface: Cliradex As a Treatment Option









By Cynthia Matossian, MD
Blepharitis affects millions of people, and Demodex mites can be one of the underlying culprits. Not only are Demodex mites linked to lid margin disease, but they can also exacerbate preexisting conditions such as DED, MGD, and rosacea blepharitis, to name a few. Unless it is addressed ahead of time, blepharitis can affect cataract and refractive surgery outcomes, disappointing patients who often have high expectations for these surgeries. One way to help patients with OSD is to include Demodex in the differential diagnosis.
Blepharitis causes itching, lid crusting, foreign body sensation, fluctuations in vision, ocular discomfort, and red, swollen eyes. Demodex is an ectoparasite that resides on skin, often harmlessly. However, when these microscopic mites multiply out of control, they contribute to blepharitis, rosacea, MGD, chalazia, and ocular surface inflammation.1-5
Clinical signs and symptoms. There are several telltale signs of Demodex: waxy collarettes around the bases of the lashes, greasy scales on the lashes and lids, thickened meibum, hair loss in the lashes or eyebrows, and cutaneous pustules around the eyes.
Educating patients with signs of Demodex. There are two schools of thought about how to approach patients with signs of Demodex. One is to epilate a lash and show the patient the mites under magnification, which can help with compliance. Without trying to scare the patient, physicians must be honest and matter-of-fact about the condition.
Another approach is to show patients the cylindrical dandruff along the eyelashes on a slit-lamp photograph. The patients can then see the telangiectasias, the lash loss, the scalloped lid appearance, and the pyramidal extensions around the base of the lash. The physician can explain that this is a common condition and that everyone has some mites but that a proliferation of these mites can cause the undesirable symptoms.

Cliradex is an all-natural lid, lash, and facial cleanser that comes in individual packets. Cliradex contains 4-Terpineol, which is reportedly more effective than tea tree oil itself in eradicating Demodex. It is also less toxic, more comfortable, and more convenient for patients than tea tree oil.6
Depending on the severity of the patient’s signs and symptoms, there are several ways to use Cliradex. For patients with severe cases of Demodex infestation, using Cliradex Complete administered in the office may be the best first step. Cliradex Complete contains a higher concentration of 4-Terpineol in a cream form. It is applied along the patient’s lash lines, brushed through the lashes, and left on for 3 to 5 minutes with the patient’s eyes closed. After the treatment, the cream is wiped off using a Cliradex wipe, and the patient is asked to purchase a box of Cliradex wipes to use once or twice daily for maintenance treatment. For less severe cases, the clinician may choose just to have the patient use the wipes at home once or twice daily for a period of 6 to 8 weeks to eradicate the mites and their eggs.
Cliradex can cause mild burning and stinging, but most patients are fine with the way it feels as long as they know what to expect. Education begins by demonstrating how to use the product in the office. The Cliradex tingle can be described to patients as uncomfortable in the beginning so that they are pleasantly surprised to find that it is not as harsh as they were expecting. It is also important that patients be instructed to keep their eyes closed until the solution has fully dried. Some patients do well by putting a cold washcloth on the face prior to using the Cliradex lid wipes.
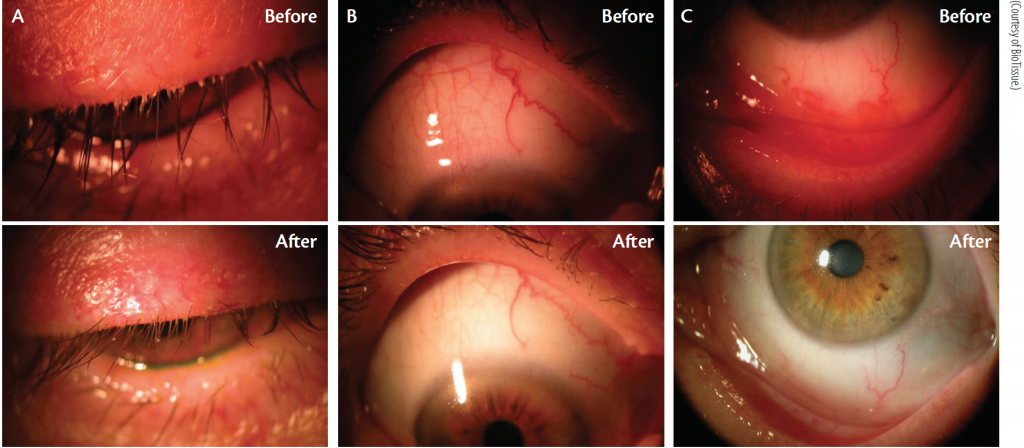
Lid margin disease with Demodex infestation is common. Cliradex can be an effective and easy-to-use treatment option for patients who experience this condition (Figure 4).
1. Cheng AM, Sheha H, Tseng SC. Recent advances on ocular Demodex infestation. Current Opin Ophthalmol. 2015;295-300.
2. Bhandari V, Reddy JK. Blepharitis: always remember Demodex. Middle East Afr J Ophthalmol. 2014;317-320.
3. Liu J, Sheha H, Tseng SC. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010;10(5):505-510.
4. Liang L, Ding X, Tseng SC. High prevalence of Demodex brevis infestation in chalazia. Am J Ophthalmol. 2014;342-348.
5. Huang Y, He H, Sheha H, Tseng SC. Ocular demodicosis as a risk factor of pterygium recurrence. Ophthalmology. 2013;1341-1347.
6. Tighe S, Gao YY, Tseng SC. Terpinen-4-ol is the most active ingredient of tea tree oil to kill Demodex mites. Transl Vis Sci Technol. 2013;2(7):2.
Cynthia Matossian, MD
• owner, founder, and chief medical officer, Matossian Eye Associates, with locations in Pennington and Hamilton, New Jersey, and Doylestown, Pennsylvania
• cmatossian@matossianeye.com
• financial interest: none acknowledged
LipiFlow Thermal Pulsation System
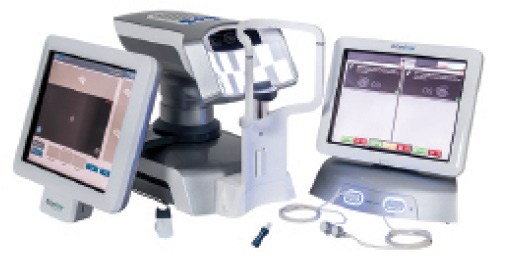

Figure 5. The LipiView and LipiFlow devices.
The LipiFlow Thermal Pulsation System (TearScience; Figure 5) is an inner and outer eyelid thermal-pulsation treatment that directly treats and unblocks the meibomian glands. LipiFlow uses vectored thermal pulsating eyepieces known as activators, which are precisely designed to provide necessary inner and outer lid contact to apply heat and to massage and evacuate the meibomian glands, allowing them to resume oil production.
The entire treatment takes about 12 minutes per eye, according to company literature.1 The eye care provider can determine how often LipiFlow should be repeated based on evaluation of the patient’s gland function and structure over time.
Controlled clinical studies have shown that, on average, LipiFlow achieves sustained threefold improvement in meibomian gland function, reducing symptoms to approximately half the preprocedure level, according to the company.
1. MGD and Dry Eye Treatment Options. TearScience. http://dryeyeandmgd.com/treatment-options/. Accessed March 14, 2016.
Use of LipiFlow in the Management of Evaporative DED











By Rohit Shetty, FRCS, PhD; and Harsha Nagaraja, MS, FCE
The prevalence of DED has been reported to range between 7.8% and 93.2% in studies across the world, with a higher prevalence in Asians.1 DED may manifest secondary to impaired lacrimal production (aqueous-deficient DED) or due to excessive evaporation (evaporative DED). Although they frequently coexist, evaporative DED is thought to be significantly more common than aqueous-deficient DED.2
MGD, characterized by terminal duct obstruction and abnormal glandular secretion, is the leading cause of evaporative DED.3 The treatment of MGD is aimed at relieving gland obstruction and restoring the normal flow of meibomian gland secretions.4 Conventionally, this is achieved through the application of heat to the glands in the form of warm compresses, heated pads or goggles, self-administered lid massage, and/or more aggressive, practitioner-administered manual expression.4
A novel thermodynamic treatment, LipiFlow Thermal Pulsation System (TearScience) combines the benefits of heat therapy and physical expression and controls the variables of temperature and pressure. Heat is applied to the palpebral conjunctival surfaces, allowing almost direct heat transfer to the underlying meibomian glands while simultaneously applying pulsatile pressure to the external lid surfaces, thereby expressing the meibomian glands.5
Before and after the LipiFlow treatment, each patient undergoes a thorough evaluation, which includes the SPEED score, number of meibomian glands yielding liquid secretions (indicative of the number of functional meibomian glands) using a handheld instrument (Meibomian Gland Evaluator), tear breakup time, lipid layer thickness using LipiView (TearScience), and Schirmer test types I and II (without and with anesthesia, respectively).
The LipiView Ocular Surface Interferometer evaluates the lipid layer of the tear film by acquiring the specularly reflected colors from the tear film. The processed output is expressed as interference color units, which is a measure of the lipid layer thickness known to correlate significantly with symptoms of DED.
In our experience, we have observed a statistically significant improvement in SPEED, the number of meibomian glands yielding liquid secretions, lipid layer thickness, and tear breakup time at 6 months posttreatment (P < .001); this was maintained up to the 12-month posttreatment visit (P ≤ .004 from baseline; P > .05 from posttreatment 6 to 12 months). Although the LipiFlow treatment is expected to improve meibomian gland function and tear film stability, tear production may remain unaffected. Hence, we found no significant change in Schirmer test findings (both I and II) at the 6- or 12-month posttreatment follow-up visits.
Patients with more than 50% of atrophic meibomian glands are not expected to respond to treatment, as LipiFlow is unlikely to lead to the regrowth of atrophic glands. Therefore, gland imaging using meibography (Keratograph 4; Oculus) to document the extent of atrophy may be a valuable tool to assess the level of structural gland compromise before treatment, to assess the urgency for treatment and to assist in setting appropriate expectations for treatment outcomes. In our experience, a single LipiFlow treatment significantly improves signs and symptoms of MGD-associated evaporative DED and is effective for at least 12 months.
1. Basak SK, Pal PP, Basak S, Bandyopadhyay A, Choudhury S, Sar S. Prevalence of dry eye diseases in hospital-based population in West Bengal, Eastern India. J Indian Med Assoc. 2012;110(11):789-794.
2. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478.
3. Nichols KK. The international workshop on meibomian gland dysfunction: introduction. Invest Ophthalmol Vis Sci. 2011;52(4):1917-1921.
4. Blackie CA, Korb DR, Knop E, Bedi R, Knop N, Holland EJ. Nonobvious obstructive meibomian gland dysfunction. Cornea. 2010;29(12):1333-1345.
5. Korb DR, Blackie CA. Restoration of meibomian gland functionality with novel thermodynamic treatment device—a case report. Cornea. 2010;29(8):930-933.
Harsha Nagaraja, MS, FCE
• Narayana Nethralaya Eye Hospital, Rajajinagar, Bangalore, Karnataka, India
• harshdr@gmail.com
• financial interest: none acknowledged
Rohit Shetty, FRCS, PhD
• Narayana Nethralaya Eye Hospital, Rajajinagar, Bangalore, Karnataka, India
• drrohitshetty@yahoo.com
• financial interest: none acknowledged
MiBo Thermoflo

Figure 6. The MiBo Thermoflo device.
The MiBo Thermoflo (MiBo Medical Group; Figure 6) is a therapeutic device for the treatment of DED. It employs a proprietary thermoelectric heat pump to help maximize liquefaction of meibum, thus improving preservation and function of the tear film’s evaporative component.
The MiBo Thermoflo supplies continuous controlled heat that is applied to the outer skin of the eyelids along with ultrasound gel for a gentle massage. The heat is absorbed deep into the tissue and breaks down the hardened lipids in the meibomian glands. With a specific prescribed therapy regimen, the ducts of the meibomian glands will secrete thinner and clearer lipids, which will allow a healthier tear film, according to company literature.1
For best results, it is recommended that physicians perform three treatments, 2 weeks apart, evaluating patients after that cycle to determine whether they require a fourth treatment. Most patients are able to go 12 weeks before requiring additional treatments, but some request frequent treatments for comfort due to daily eyestrain, according to the company.
1. MiBo Thermoflo. MiBo Medical Group. http://mibomedicalgroup.com. Accessed March 14, 2016.
MiBo Thermoflo: An Essential Tool for Anterior Segment Surgeons
By James S. Lewis, MD









MiBo ThermoFlo safely manages perioperative DED from MGD (Figure 7). Blepharitis and tear deficiency frequently complicate surgical results in LASIK, cataract surgery, and corneal transplantation. An otherwise perfect surgery, compromised by mild DED-related visual fluctuation, can cost the busy practitioner significant chair time and may degrade patients’ confidence. MiBo treatment is quick, convenient, comfortable, and focused on the underlying pathology. More important, MiBo can also address moderate to severe tear film deficiency threatening both acuity and ocular health. In my opinion, this device belongs in the repertoire of the busy anterior segment surgeon.
MiBo is a low-cost adjunct to premium and standard cataract surgery. Because lipid-deficient DED dominates this demographic, pre- and postoperative MiBo treatments can prevent problems and enhance patients’ satisfaction. When warm compresses and patient-centric lid hygiene regimens fail, in-office therapy with MiBo can improve patients’ care and clinical results. Theoretically, patients’ safety is enhanced by decreasing excessive lipids and their associated bacterial load. I routinely administer MiBo to cataract patients with excessive lid disease preoperatively and often at least once in the postoperative period. The device is extremely helpful when patients claim that they are not seeing as well as they had hoped and the tear film is the cause.
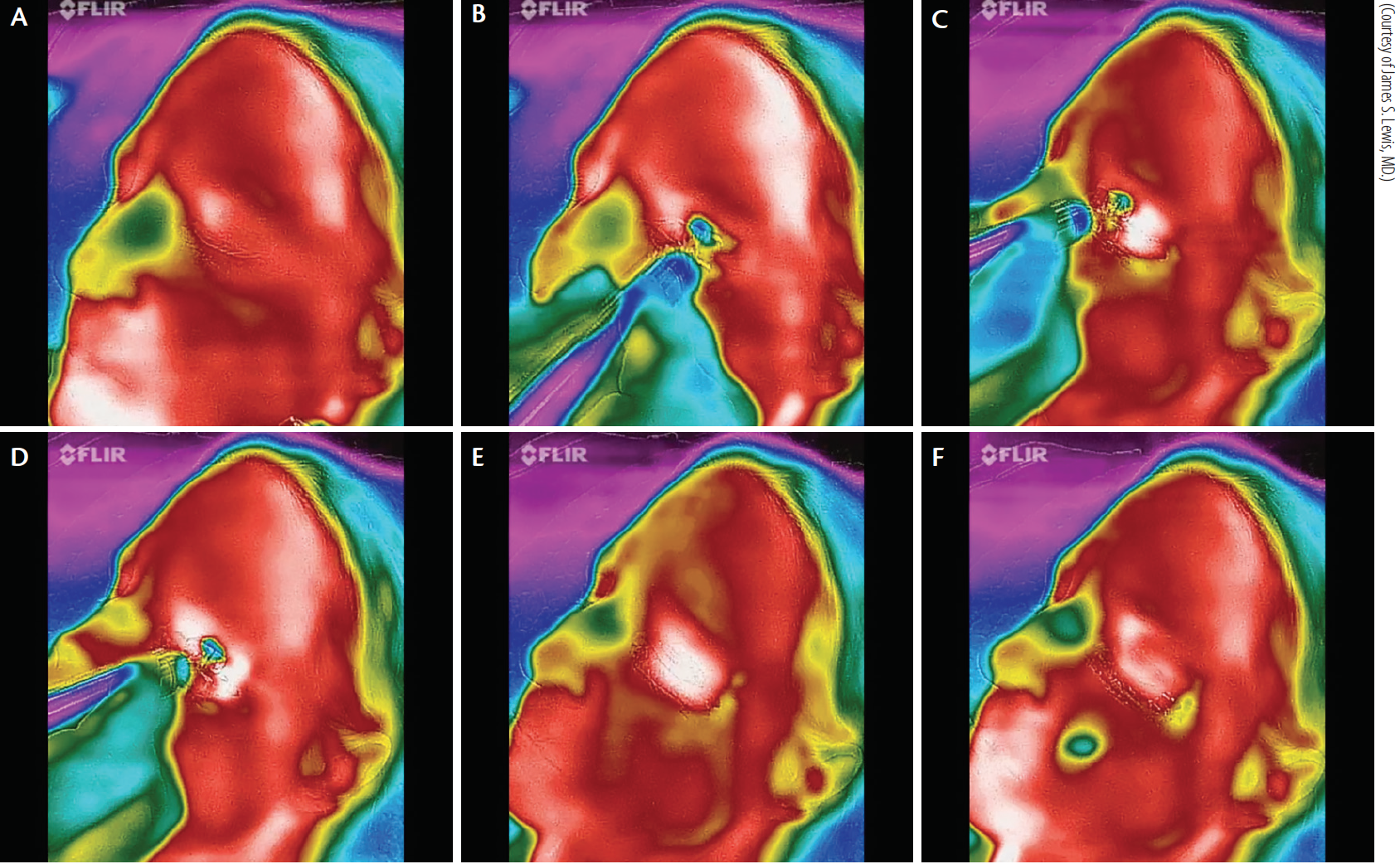

Figure 7. Thermal depiction of MiBo Thermoflo on an infrared camera with pseudo-color encoding: before treatment (A). The red skin temperature is warmer than the green nasal passages (B). MiBo heats the upper and lower lid skin to a hot white color (C). The entire meibomian gland apparatus is elevated to 108ºF (D). After removal of MiBo’s emissive heat, the lid remains warm (E). More than 1 minute later, a therapeutic effect is seen (F).
LASIK patients benefit from MiBo even when their MGD is minimal or subclinical. The tear film disruption inherent in the procedure responds well to this treatment. I have performed MiBo on LASIK patients within 48 hours of the procedure. In my experience, MiBo treatments hasten re-epithelialization after epi-LASIK and PRK and also help those undergoing flap lifts. Visual fluctuation resulting from changes in corneal architecture and interruption of the nerves is well addressed by this treatment.
I find MiBo useful before and after all types of penetrating keratoplasty. I have used MiBo in the first postoperative week following Descemet stripping endothelial keratoplasty and Descemet membrane endothelial keratoplasty. For neglected patients with prolonged epithelial edema prior to surgery, it can take weeks to recover an acceptable surface. MiBo has been helpful in this clinical scenario as well as in full-thickness keratoplasty and deep anterior lamellar keratoplasty.
Although most clinicians will use MiBo as an inexpensive, convenient, and effective therapy for MGD and associated DED, I find it indispensable in the management of a diverse collection of anterior segment surgical procedures. In my experience, MiBo decreases infection risk preoperatively and hastens epithelial regrowth, thereby reducing the risk of scarring in the early postoperative period. Equally helpful is MiBo’s ability to enhance the tear film following LASIK and premium IOL surgery for patients’ comfort. Optimizing ocular surface health helps to control fluctuating vision and bolster patients’ satisfaction, thereby promoting practice growth.
I temporarily lost access to my MiBo this past year when I sent it back to the company for an upgrade. Only then did I realize how dependent I had become on this seemingly simple but efficient device. MiBo is an essential tool for the anterior segment surgeon. n
James S. Lewis, MD
• private practice, Elkins Park, Pennsylvania
• jslewis@jameslewismd.com
• financial disclosure: small ownership in MiBo Medical Group


